News — Today, most of us carry a fairly powerful computer in our hand—a smartphone.
But computers weren’t always so portable. Since the 1980s, they have become smaller, lighter, and better equipped to store and process vast troves of data.
Yet the silicon chips that power computers can only get so small.
“Over the past 50 years, the number of transistors we can put on a chip has doubled every two years,” said , assistant professor of physics at the University of Miami . “But we are rapidly reaching the physical limits for silicon-based electronics, and it’s more challenging to miniaturize electronic components using the we have been using for half a century.”
It’s a problem that Wang and many in his field of molecular electronics are hoping to solve. Specifically, they are looking for a way to conduct electricity without using silicon or metal, which are used to create computer chips today. Using tiny molecular materials for functional components, like transistors, sensors, and interconnects in electronic chips offers several advantages, especially as traditional silicon-based technologies approach their physical and performance limits.
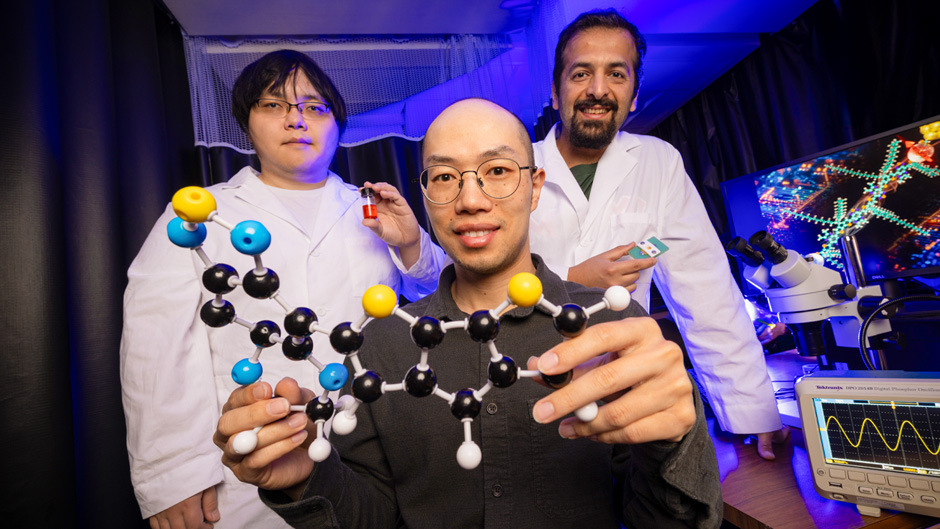
But finding the ideal chemical makeup for this molecule has stumped scientists. Recently, Wang, along with his graduate students, Mehrdad Shiri and Shaocheng Shen, and collaborators Jason Azoulay, associate professor at Georgia Institute of Technology, and Ignacio Franco, professor at the University of Rochester, uncovered a promising solution.
This week, the team shared what they believe is the world’s most electrically conductive organic molecule. Their discovery, published in the , opens up new possibilities for constructing smaller, more powerful computing devices at the molecular scale. Even better, the molecule is composed of chemical elements found in nature—mostly carbon, sulfur, and nitrogen.
“So far, there is no molecular material that allows electrons to go across it without significant loss of conductivity,” Wang said. “This work is the first demonstration that organic molecules can allow electrons to migrate across it without any energy loss over several tens of nanometers.”
The testing and validation of their unique new molecule took more than two years.
However, the work of this team reveals that their molecules are stable under everyday ambient conditions and offer the highest possible electrical conductance at unparalleled lengths. Therefore, it could pave the way for classical computing devices to become smaller, more energy-efficient, as well as cost-efficient, Wang added.
Currently, the ability of a molecule to conduct electrons decreases exponentially as the molecular size increases. These newly developed molecular “wires” are needed highways for information to be transferred, processed, and stored in future computing, Wang said.
“What’s unique in our molecular system is that electrons travel across the molecule like a bullet without energy loss, so it is theoretically the most efficient way of electron transport in any material system,” Wang noted. “Not only can it downsize future electronic devices, but its structure could also enable functions that were not even possible with silicon-based materials.”
Wang means that the molecule’s abilities might create new opportunities to revolutionize molecule-based quantum information science.
“The ultra-high electrical conductance observed in our molecules is a result of an intriguing interaction of electron spins at the two ends of the molecule,” he added. “In the future, one could use this molecular system as a qubit, which is a fundamental unit for quantum computing.”
The team was able to notice these abilities by studying their new molecule under a scanning tunneling microscope (STM). Using a technique called STM break-junction, the team was able to capture a single molecule and measure its conductance.
Shiri, the graduate student, added: “In terms of application, this molecule is a big leap toward real-world applications. Since it is chemically robust and air-stable, it could even be integrated with existing nanoelectronic components in a chip and work as an electronic wire or interconnects between chips.”
Beyond that, the materials needed to compose the molecule are inexpensive, and it can be created in a lab.
“This molecular system functions in a way that is not possible with current, conventional materials,” Wang said. “These are new properties that would not add to the cost but could make (computing devices) more powerful and energy efficient.”